Inogen: The case of dangerous Oxygen
Not investment advice. Article is strictly author's opinion only. The Nexus Files is a self-funded journalistic publication based in Europe
Introduction
Inogen is a company which sells lightweight oxygen concentrators for patients. This report’s primary concern regards the safety of these Oxygen Concentrators.
Every once in a while, there comes along a medical company engineered for profit companies— think Valeant Pharma, or Insys therapeutics-—with perverse intentions to reap profits and harming lives in the process.
Could Inogen be the latest breed of such companies?
To begin with, in the past, the FDA has recalled Oxygenic products, due to only a few complaints, but Inogen’s case proves to be even more bizarre. There have been cases of severe injuries and hospitalization due to use of Inogen’s products and a few defects that have even caused oxygen leaks, which have caused explosions.
Another case of an oxygenic device recall by the FDA is:
‘Several reports’ of fires? Well, Inogen’s problems extend to more than what can be encompassed by the word ‘several’.
Method of investigation
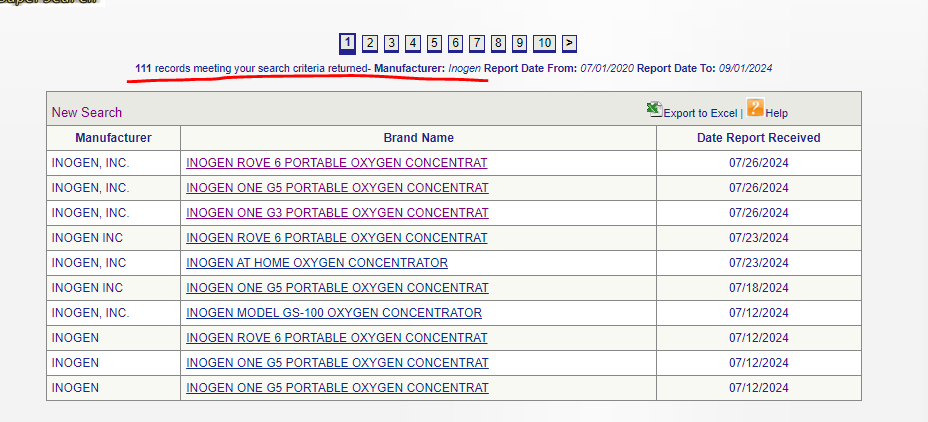
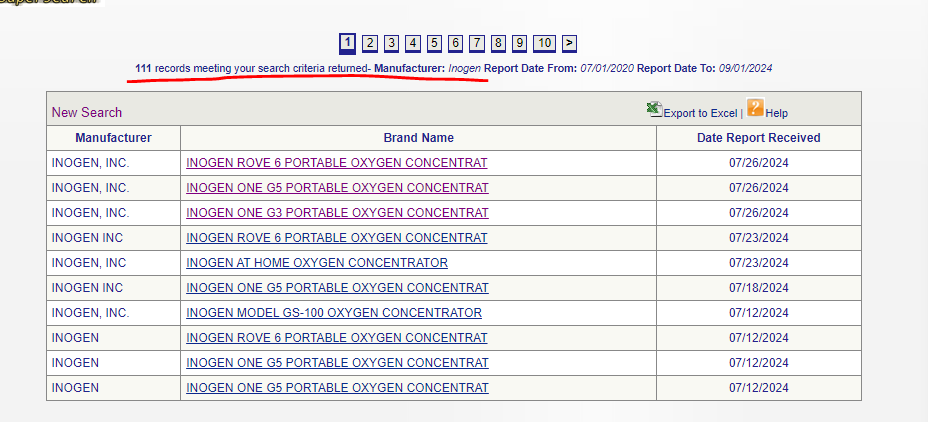
The primary way of vetting Inogen’s products is through the MAUDE database, procured by the FDA:
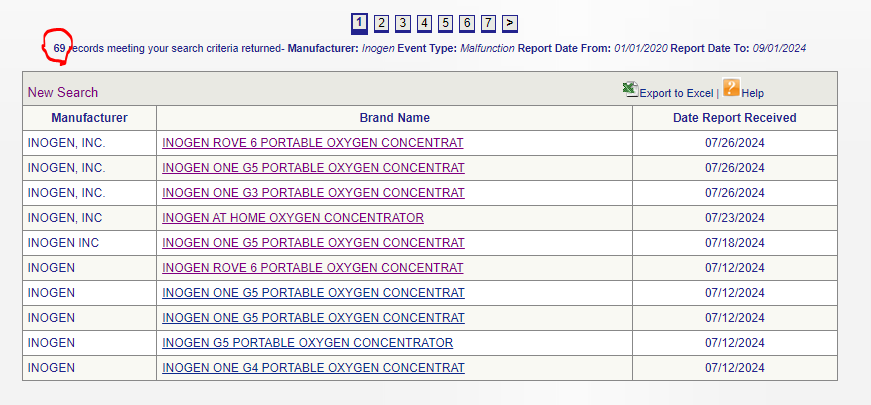
This is a list of all adverse events, injuries, deaths, and malfunctions caused by the device. As previously referenced, the FDA has forcefully taken various devices off the market due to some milder complaints and injuries. Inogen, however, has been the subject of 111 adverse event reports since 2020.
The Hospitalization Cases
Any adverse event, especially in the case of an oxygen tank, can trigger the FDA to start looking at Inogen and trigger a recall—and we reckon that the FDA would be shocked to see the sheer volume of hospitalization cases, fires, and defects that are manifesting through Inogen’s oxygen concentrators.
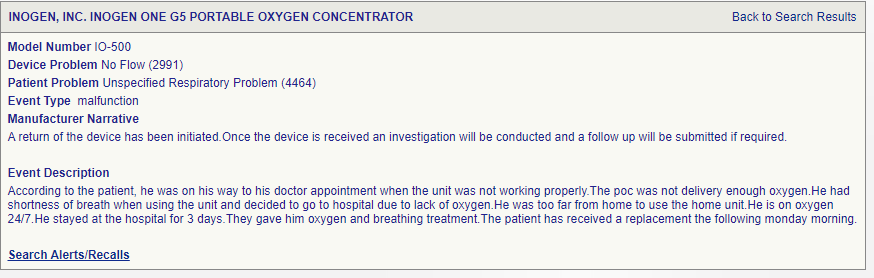
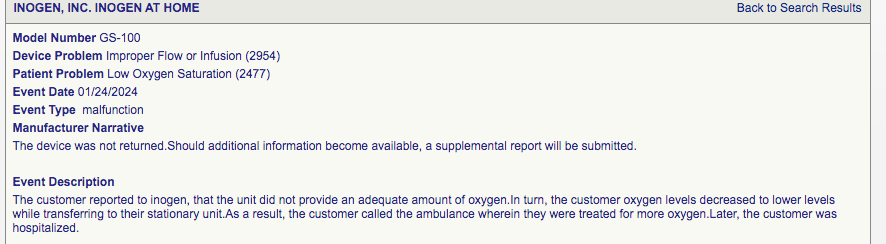
26th July 2024. Unit stopped delivering adequate oxygen
26th July 2024. Inogen device suddenly stops supplying oxygen
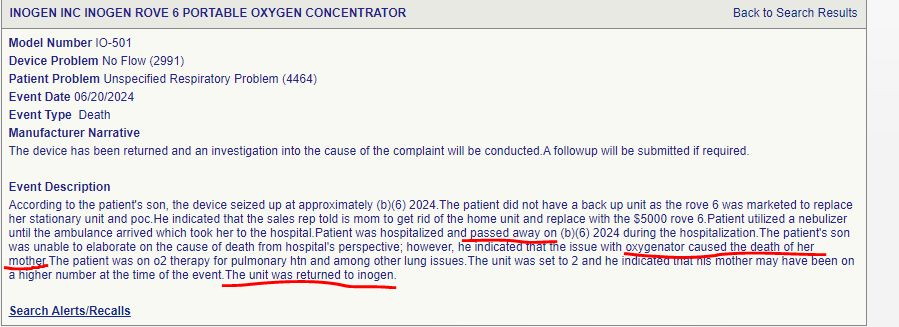
Death due to issue with oxygenator. Dated 20th August 2024
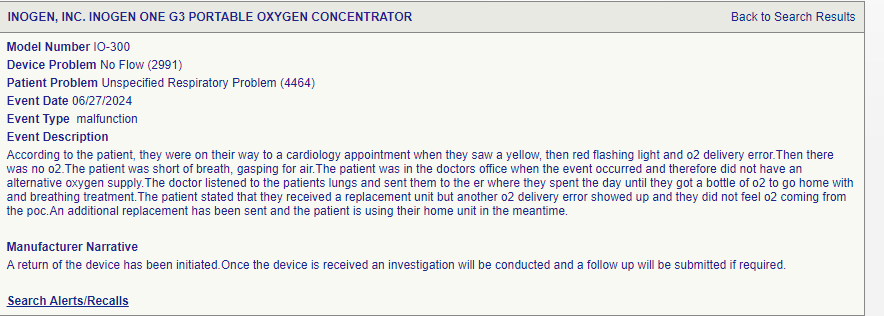
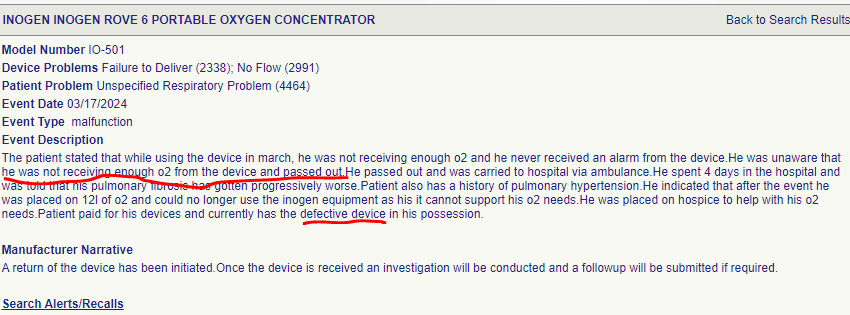
17th March 2024. Patient with defective Inogen device passes out
Another case:
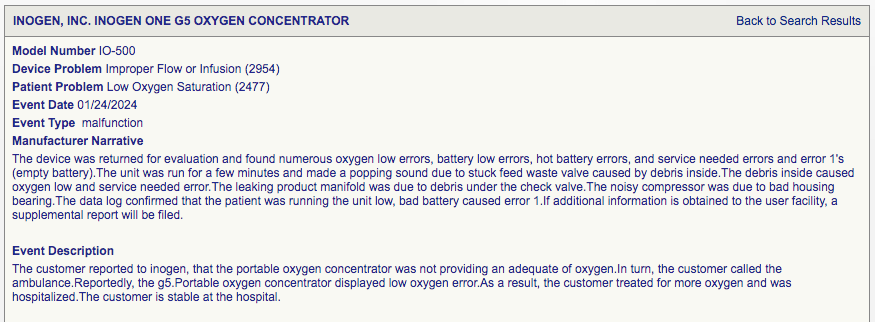
24th January 2024- Unit stops providing adequate oxygen, and patient had to call an ambulance
The fact that Inogen’s devices have already been the cause of an overwhelming number of patient injuries and malfunctions reinforces our theory that Inogen’s 70% stock increase in the last 6 months is unwarranted, so we call on the FDA to take action.
One of the most bizarre cases, however, is:
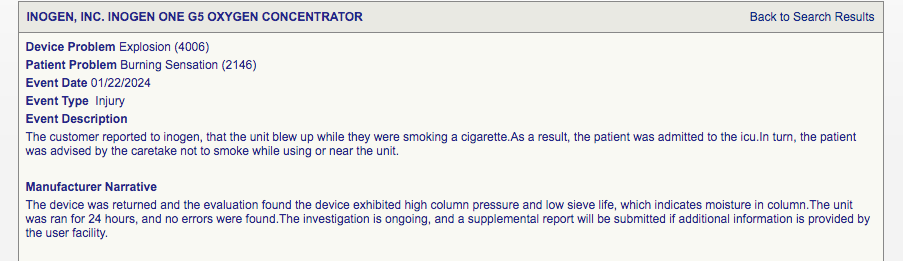
Oxygen concentrator explodes
It is common knowledge that lighting a cigar near an oxygen concentrator is not a good idea. However, the fact that an explosion occurred due to low sieve life and moisture in the column is simply a testament for low product efficacy—and a completely wrecked safety profile.
All in all, there are about 69 cases, since 2020, of device malfunctions, including showing false battery meters, or just shutting the oxygen flow, resulting in patients being rushed to the hospital via an ambulance.
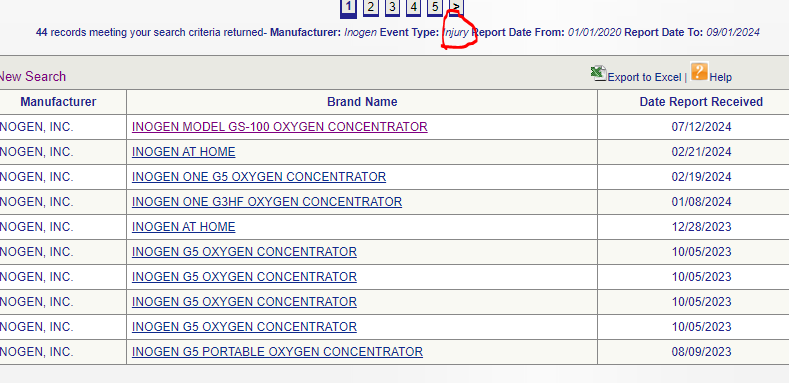
However, that is not all. ‘Malfunctions’ and ‘Injuries’ are counted separately in the MAUDE database. The amount of injuries are:
44.
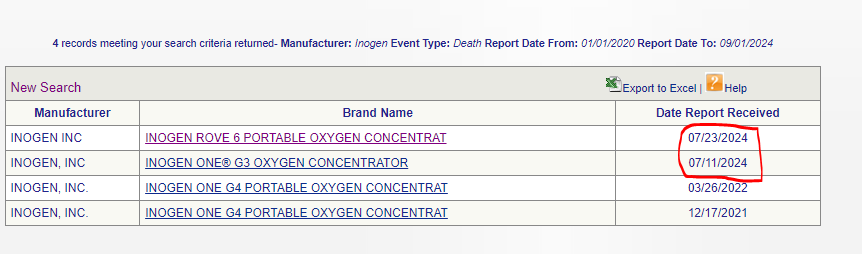
Next, we obviously have Inogen’s death profile:
Two alone in 2024.
Extremely concerning to say the least. Our guess is that Inogen knows this very well. Companies have the option to voluntarily take their products off market via an FDA recall—but why would they do this ?
This would be devastating to management’s equity—and the company’s revenues!
Corruption at its finest?
The case for deception:
While the accounts on the FDA MAUDE database are anonymous, they are very educating:
Source
It seems to us like Inogen has a severe problem with its oxygen concentrator, and it is gambling with lives.
Okay, we got the main smoking gun: destroying lives due to greed. But, what about the work of other activists?
4In 2019, activist short seller Muddy Waters Research (https://www.muddywatersresearch.com/research/ingn/mw-short-inogen/), exposed Inogen’s farcical claims about it’s TAM (Total Addressable Market)—where Inogen made absurd claims about the growing Oxygen concentration market—just to pump up the stock price.
Muddy’s report was eye-opening to the public markets because it revealed that INGN’s management was solely to portray itself as a high-growth medical device company … a narrative mostly taken by companies to pump up the stock price and enrich insiders.
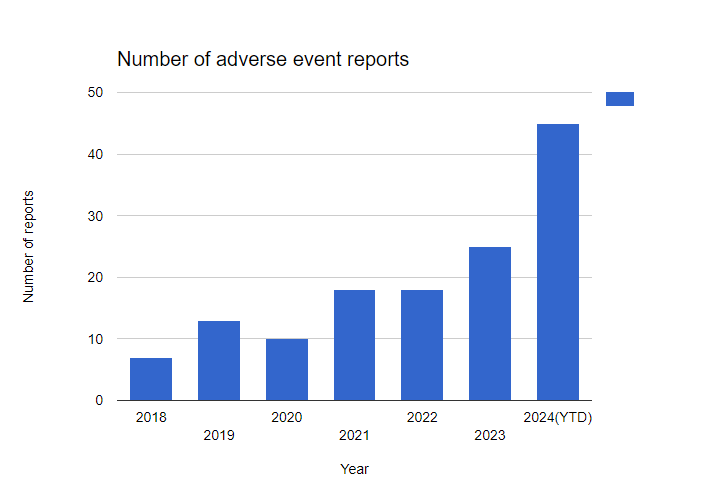
Growing adverse events on the FDA MAUDE database: Visualised:
Note: We only used the number of adverse events filed from Jan 1st 2024 to September 2nd 2024! The number of adverse event reports is at a yearly all-time-high … and the year hasn’t even finished!
Problems being caused by Inogen’s devices are ramping up, and customers, such as hospitals and patients really ought to stop using devices that threaten lives as blatantly as Inogen. The number of adverse events is outrageous.
The FDA is known to be strict with product recalls—and it has taken products off market for barely 5-6 injuries.
What’s the bulk of problems from the FDA MAUDE database logs?
The main problems rising from Inogen’s concentrators are a lot of battery, supply, and valve errors. ‘Pops’ and explosions are caused by leakages from the product—and a lot of battery and supply errors are just design flaws—albeit serious enough to kill a patient or at the very least warrant an urgent ambulance ride to the hospital.
Muddy Water’s accusations: Misleading investors about hype to boost stock price
While the actual report is multiple pages long, a succinct summary is:
https://www.muddywatersresearch.com/research/ingn/mw-short-inogen/
Inogen’s stock is down 94% from its publishing date (Feb 8th 2019)—basically vindicating Muddy’s accusations.
Now, pulling behind the curtains, one thing is clear: the main objective of management is to make money, not to save lives.
Overstating TAM (Total Addressable Market) is something that is done ONLY to increase stock price—enriching the net worths of the CEO and other executives.
Who’s to say that Inogen isn’t doing this now ?
It is the responsibility of a company to take appropriate measures to ensure the safety of its products, yet Inogen—a company that doesn’t sell drugs or medicines, but instead Oxygen concentrators, a life-essential for affected patients—is not engaging in the industry standard when it comes to both ethics and protocol.
Inogen’s concentrators FAIL (well, sort of) the FDA’s objectives on allowing a 510(k) premarket approval
In order to get a device approved, there are 2 ways to get approval from the FDA:
The right way: Device goes through adequate testing and it is decided by the FDA whether the device is safe or not.
The swift way: The 510(k) approval method—with its only objective being to prove that its safety profile is similar to a device already in the market. Companies use this when they have incentive to hurry and just put poorer products on the market.
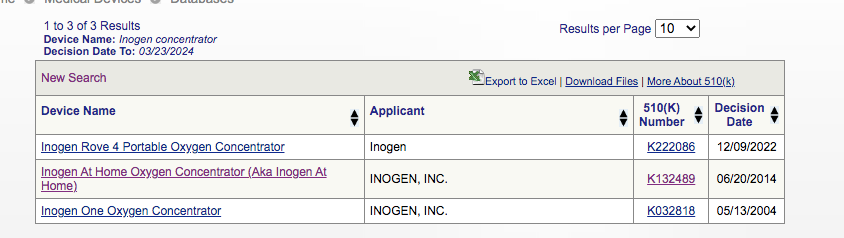
Inogen has the following concentrators through the 510(k) clearance:
Now, we decided to compare the safety profiles of Inogen’s concentrators, and the concentrators their safety profiles were compared with in order to grant FDA clearance.
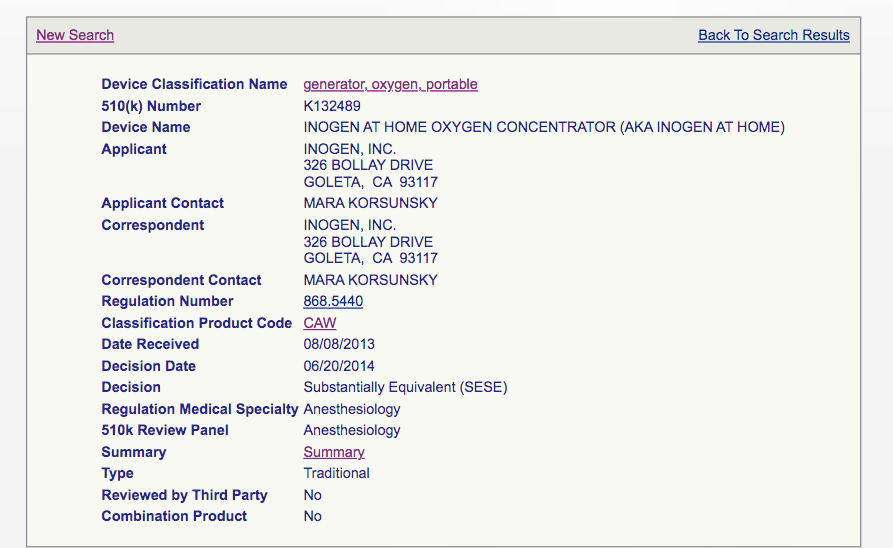
Now, the Inogen ‘At Home’s Oxygen’ concentrator’s 510(k) profile shows:
Clicking ‘summary’ redirects us to (https://www.accessdata.fda.gov/cdrh_docs/pdf13/K132489.pdf):
“Respironics L4 Oxygen Concentrator”. Basically, Inogen’s at-the-home concentrator was approved assuming its safety profile was similar to that of the highlighted device.
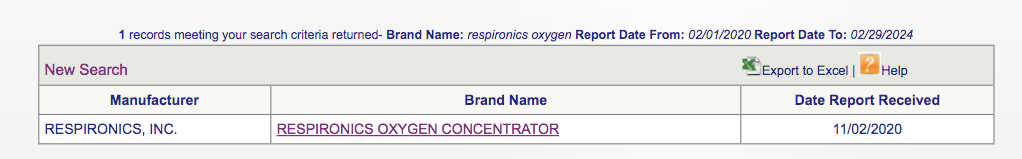
Something bizarre? Respronics’ Oxygen concentrators (excluding it’s ventilators) only have had 1 Medical device report since 2020:
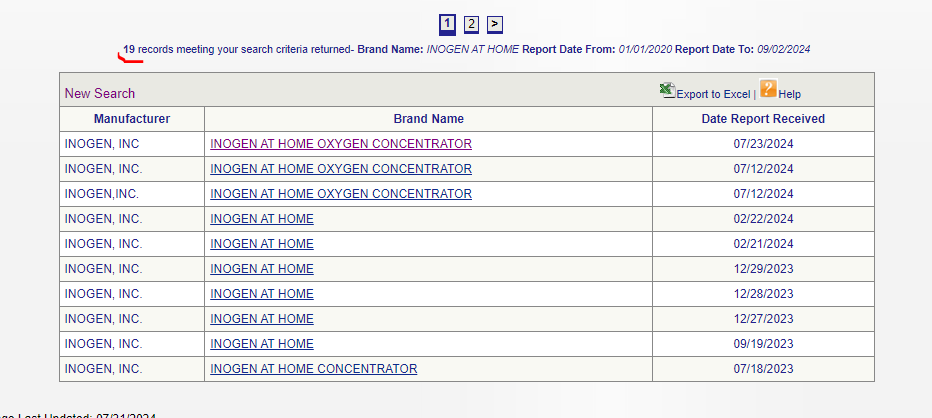
What about Inogen, in contrast?
AT LEAST 16
How can the FDA allow for such egregiousness ?
Note: The acknowledgement of the problems with the FDA and the ‘Inogen-at-the-home’ is only to showcase the negligence of the FDA. The whole point of the 510(k) fastrack approval is to prove that the safety profile is similar to another device already in the market—but the fact that the AT-THE-HOME device of Inogen has caused 19x the amount of problems just is a testament of how poorly Inogen is handling their job.
Besides, the at-the-home concentrator accounts for only part of the problem. For instance, since 2020, there were 111 Medical Device reports:
Most of those stemming from the ‘G5 Concentrator’, which was given the regular approval.
If we take the broad number of Medical device reports pertaining to Oxygen generators from the FDA database, we see that an unhealthy amount pertains to Inogen.
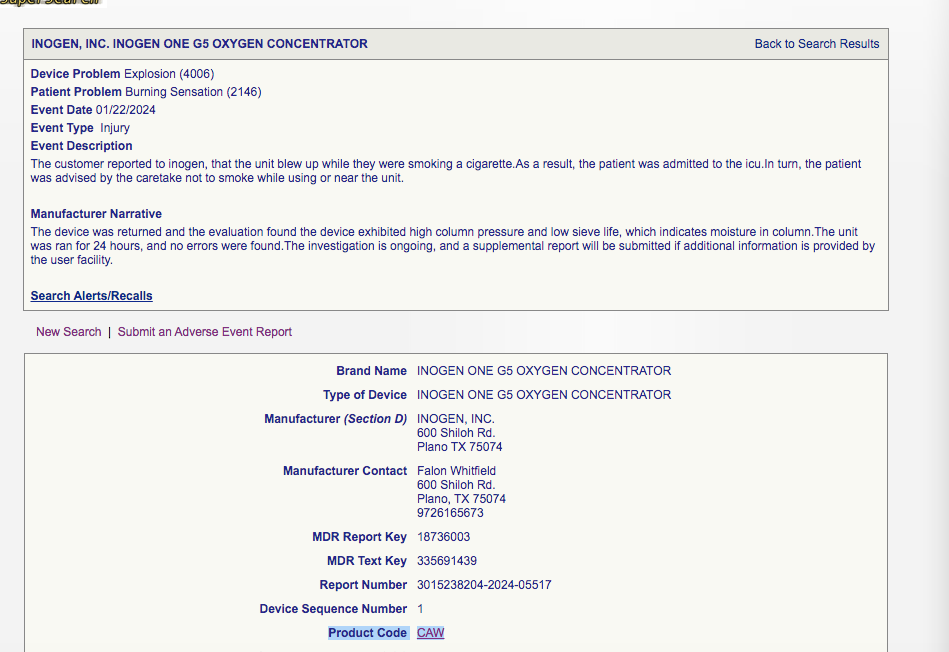
If we visit any single Medical Device Report on the FDA MAUDE database (say, https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/detail.cfm?mdrfoi__id=18736003&pc=CAW&device_sequence_no=1):
We see ‘product code CAW’.
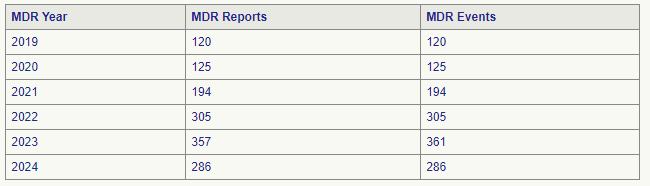
The FDA groups medical devices into ‘product codes’ so we can see grouped statistics altogether. For example, the TPLC (Total Life Cycle) report for product code ‘CAW’ (this is basically a report revealing how many Medical Device Reports there were for oxygenic products TOTALLY—not just for Inogen), we can see that:
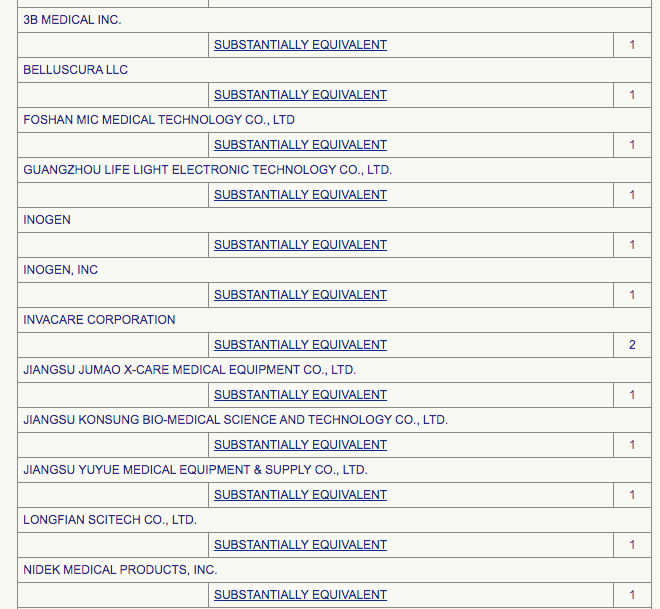
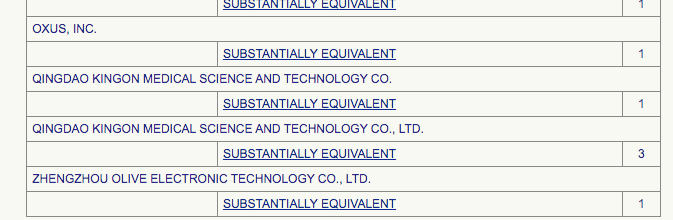
MDR stands for ‘Medical Device Reports’. The oxygen concentrators included in this tally are:
Basically, the Medical Device reports are spread between 16 oxygenic products.
Here is something disturbing: we already know that Inogen has had about 111 MDRs since 2020. The TPLC report says that since 2020, there were 125 + 194 + 305 + 357 + 286 = 1267 MDRs.
This means that Inogen ALONE was responsible for about 8% (very, very, very bad concentration) of all the accidents involving oxygenic devices since 2020, as reported by the FDA MAUDE.
This is egregious.
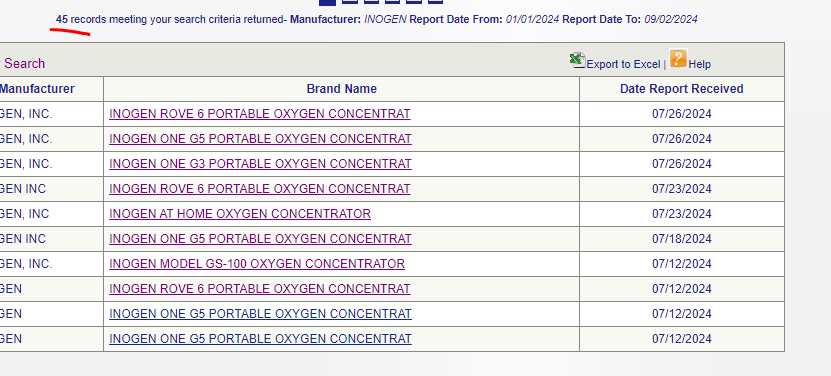
We know that in 2024, INGN produced 45 adverse event reports:
Look at the figures for 2024. Inogen’s devices reported about 45 MDRs in 2024, and the total oxygenic landscape gave rise to about 286 MDRs in 2024—meaning that Inogen accounted for more or less an egregious 15% of total oxygenic adverse events involving oxygen concentrators.
The numbers have really gotten worse in 2024.
We think that this is an example of a company using shareholders’ funds to make defective products which have started to cost lives.